The chemical reaction systems unit test delves into the intricate world of chemical reactions, providing a comprehensive examination of their definitions, types, and factors influencing their rates. This test serves as a gateway to understanding the principles governing chemical transformations and their profound implications in various scientific disciplines.
By exploring the concepts of activation energy, catalysts, and equilibrium, the test challenges students to grasp the dynamics of chemical reactions. It also sheds light on the energy changes associated with these processes, highlighting the significance of enthalpy and entropy.
Furthermore, the test delves into redox reactions and electrochemistry, unraveling the mechanisms underlying electron transfer and electrochemical cell functioning.
Chemical Reactions: Definitions and Concepts: Chemical Reaction Systems Unit Test
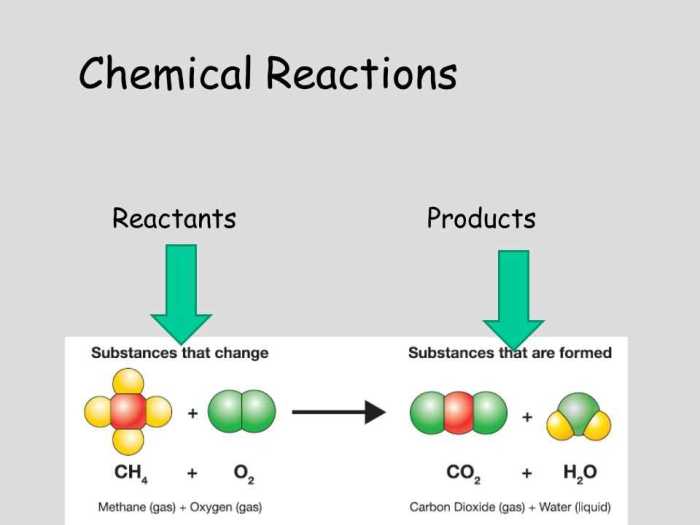
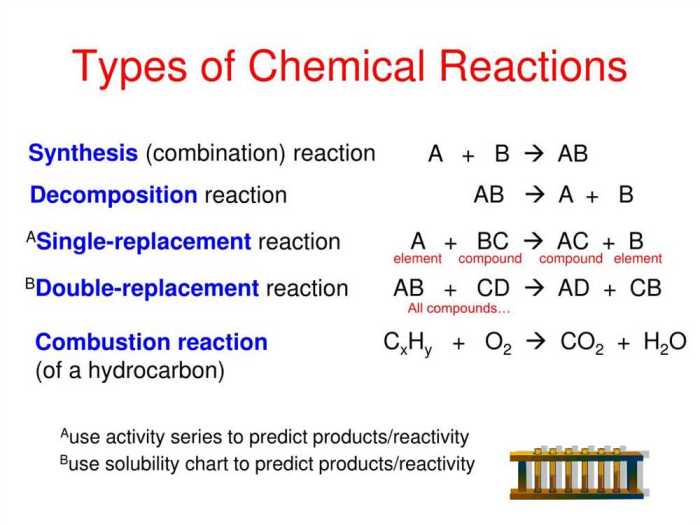
Chemical reactions involve the rearrangement of atoms and molecules to form new substances. They can be classified into various types based on their characteristics, including combination, decomposition, single displacement, double displacement, and combustion reactions. Examples include the formation of water from hydrogen and oxygen, the decomposition of calcium carbonate to form calcium oxide and carbon dioxide, and the displacement of copper from copper sulfate solution by iron.
Reaction Rates and Factors Affecting Them
The rate of a chemical reaction depends on several factors, including the concentration of reactants, temperature, surface area, and the presence of a catalyst. Activation energy refers to the minimum amount of energy required for a reaction to occur. Catalysts are substances that increase the rate of a reaction without being consumed.
Equilibrium and Le Chatelier’s Principle
Chemical equilibrium is a state in which the forward and reverse reactions occur at the same rate, resulting in no net change in the concentrations of reactants and products. Le Chatelier’s principle states that if a change is applied to a system in equilibrium, the system will shift in a direction that counteracts the change.
Examples include the effect of adding heat on the equilibrium of an exothermic reaction.
Thermochemistry and Energy Changes
Enthalpy is a measure of the heat content of a system, while entropy is a measure of disorder. Chemical reactions can be exothermic (release heat) or endothermic (absorb heat). Hess’s law allows for the calculation of the enthalpy change of a reaction by combining the enthalpy changes of individual steps.
Redox Reactions and Electrochemistry, Chemical reaction systems unit test
Redox reactions involve the transfer of electrons between atoms or ions. They can be classified as oxidation-reduction reactions, in which one substance is oxidized (loses electrons) and another is reduced (gains electrons). Electrochemical cells utilize redox reactions to generate electricity or drive chemical reactions.
Applications of Chemical Reactions
Chemical reactions play a crucial role in everyday life, from the combustion of fuels to the production of pharmaceuticals. They have applications in industries such as medicine, agriculture, and manufacturing. However, it’s important to consider the environmental impact of chemical reactions and strive for sustainable practices.
Common Queries
What is the significance of activation energy in chemical reactions?
Activation energy represents the minimum energy required for a chemical reaction to occur. It determines the rate at which reactions proceed, with higher activation energies leading to slower reactions.
How does Le Chatelier’s principle predict the behavior of chemical reactions at equilibrium?
Le Chatelier’s principle states that if a stress is applied to a system at equilibrium, the system will shift in a direction that relieves the stress. This principle helps predict the effects of changes in concentration, temperature, and pressure on equilibrium reactions.
What are the applications of redox reactions in everyday life?
Redox reactions play a crucial role in numerous processes, including combustion, photosynthesis, and the functioning of batteries. They are also essential in industrial processes such as metal refining and the production of chemicals.